Currently, the Drugs Controller General of India (DCGI) acquiesced DRDO’s 2-DG drug for treating Covid19, which collaborated with Dr Reddy’s laboratory. However, from the side of DRDO and Dr Reddy’s lab, they still do not release any research article for this drug’s potential. This information is only collected from the News and online sources. Then What happened? Why is it acquiesced to treat covid patients? Let us see the complete report!
2-DG drug:
The 2-DG drug is modified glucose that gives potential survival chances and claims Defence Research & Development (DRDO). The Institute of nuclear medicine produced this analogue of glucose, and allied science (INMAS) is one of the groups in DRDO collision with Dr Reddy’s laboratory. Since it has been available for a long time ago, it primarily acts as an anticancer and anti-inflammatory drug; during the first wave of covid19 DRDO-INMAS and Centre for cellular and molecular biology (CCMB) in Hyderabad conducted a laboratory test for the 2-DG drug in April 2020, which showed effective results on coronavirus.
Clinical trials:
In May 2020, 2-DG got approved for Phase II trial by DCGI and Central drug standard control organisation (CDSCO). It was conducted on 110 patients all over the country—Phase IIa in six hospitals and phase IIb in 11 hospitals. Phase II results showed limited oxygen requirement (2.5 days little) compared to standard of care, which cures the disease faster.
The favourable results of phase 2 DCGI approved the phase III trial in November 2020; it was conducted in 27 hospitals from December 2020 to March 2021. In May 2021, Defence Research & Development organisation acquiesced from the beneficial results of trials. 2-DG also aids in Emerge critical use among covid19 patients. Since the DCGI released the first batch of 2 DG sachets, the second batch will start from the first week of June 2021 and be released in mid-June. Dr Reddy’s laboratory fixed the price of one sachet of 2-DG at Rs 990.
Mechanism:
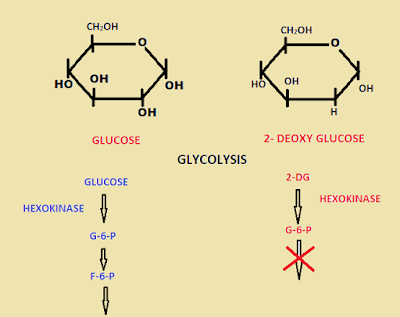
2-DG is 2-Deoxy D Glucose that acts as an anticancer and anti-inflammatory drug and acts as an antiviral drug. Nevertheless, we have to know how it works on cancer patients. This analogue of glucose inhibits the glycolysis process, which is the initial step of metabolism and produces a tiny amount of energy. The phenomenon in cancer patients, cancer cells divide rapidly and spread all over the body. In the case of 2-DG treated cancer patients, the drug inhibits the glycolysis and halts the process of metabolism So that the malignant cells can’t divide into two without energy. Thus, it acts as an anticancer and inflammatory drug. Here comes the point we use the 2-DG for Covid treatment. Virally infected cells cause reprogramming in a metabolic process that enhances glycolysis and glycosylation to facilitate viral replication and infection. As it inhibits glycolysis, it suppresses viral replication and illness and also reduces the oxygen requirement. There is a stumbling block for this treatment. The 2-DG is oral and can be spread all over the body. So the normal cells also affected by this treatment, except the liver and kidney, cannot uptake the dissolved 2-DG.
When should a covid-19 patient can take this drug?
From the study of this drug, it is used for moderate to severe covid19 patients. So it can reduce the oxygen requirements for the affected patients. It should be used only after a doctor’s prescription.
Though we are confused about covid19 drugs, this may be a feasible option in aiding the treatment of covid patients. Therefore, We can conclude that 2-Deoxy glucose can be used to adjunct the covid treatment effectively.
Discover more from Aristoscienceworld
Subscribe to get the latest posts sent to your email.