Amino acids are the building block of protein, the same as the brick needed for a house. The different combinations of amino acids produce different classes of proteins like hormones, enzymes, growth factors, transporters, antibodies, antibiotics, mushroom poison, milk protein, horn, eye lens and hair. This article comes up with a brief explanation of different classes of amino acids and their significance.
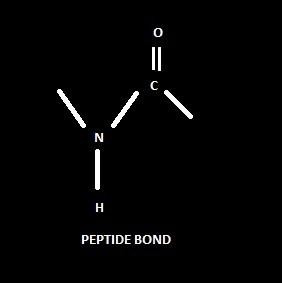
Amino acids are monomeric molecules that join with other amino acids through covalent bonding. During the attachment to the other amino acid, a bond is formed called a Peptide bond. For each formation of peptide bonds, one molecule of water (H2O) will be eliminated. Similarly, during the breakdown of protein, hydrolysis occurs, in which one molecule of H2O is attached.
The 20 different amino acids form millions of proteins in different classes. The first identified amino acid was Asparagine in 1806, and the last discovered amino acid was Threonine in 1938.
Structural features of amino acids:
Amino acids are common in structural aspects; literally, they differ by the R group’s side chain. They have a carboxyl group (COOH) and an amino group (NH2) attached to the same carbon atom, which is α- carbon (chiral carbon). The side chain of the amino acids differs in size and electric charge—these influence the amino acids to be soluble in water.
Classification of amino acids:
Amino acids are classified based on side chain (R group). Note: Every amino acid is denoted with a Single letter for easy understanding in sequencing and experimental studies.
Non-polar, Aliphatic R group:
Glycine (GLY) → G
Alanine (ALA) → A
Valine (VAL) → V
Leucine (LEU) → L
Isoleucine (ILE) → I
Proline (PRO) → P
Methionine(MET) → M
Polar, uncharged R group:
Serine (SER) → S
Threonine (THR) → T
Cysteine (CYS) → C
Asparagine (ASN) → N
Glutamine (GLN) → Q
Aromatic R group:
Phenylalanine (PHE) → F
Tyrosine (TYR) → Y
Tryptophan (TRP) → W
Positively charged R group:
Lysine (LYS) → K
Arginine (ARG) → R
Histidine (HIS) → H
Negatively charged R group:
Aspartate (ASP) → D
Glutamate (GLU) → E
Properties:
Learning the structure and property of amino acids are necessary for biochemical studies. The interaction between the amino acid and water will be the main property of amino acids, i.e. polarity of the amino acids side chain. The polarity of the amino acids are widely classified as hydrophobic (water-hating) and hydrophilic (water-loving). These two play a significant role in amino acids properties in protein structure and function. Single amino acid substitutions or deletion or insertion may primarily affect the protein functions.
Non-polar, Aliphatic R group:
This class of amino acids are nonpolar which is hydrophobic in nature. Glycine has the shortest and simplest side chain that primarily contributes to the turns or loop for connecting the protein. Alanine, valine, leucine and isoleucine have side chains responsible for stabilizing the protein by their hydrophobic interactions. Methionine has the sulfur atom in its side chain. Note that methionine doesn’t form disulfide bonds because the sulfur got covered by methyl groups in methionine. Finally, proline (imino acid) has a cyclic structure meaning that the secondary amino group of the amino acid forms a rigid cyclic structure with a side chain. Therefore proline decreases the flexibility of the protein.
Polar, uncharged R group:
This type of protein belongs to the polar that is hydrophilic (water-loving) nature. These amino acids have the side chain that forms hydrogen bonds with water. Polarity in serine and threonine is due to their hydroxyl group in the side chain, cysteine by its sulfhydryl group and asparagine and glutamine by their amide group. Asparagine and glutamine are amide derivatives of aspartate and glutamate, respectively which means they are sensitive against acid or base. Cysteine is formed by oxidation, in which two cysteine molecules are linked by a disulfide bond and forms the dimeric cysteine amino acid. This disulfide bond is hydrophobic in nature and plays a role to attach two different polypeptide chains covalently.
Aromatic R group:
This class is relatively hydrophobic in nature and has an aromatic functional group. Tyrosine hydroxyl group and nitrogen in the tryptophan’s indole ring make tyrosine and tryptophan more polar than phenylalanine. As a result, the tyrosine hydroxyl group can form a hydrogen bond. Phenylalanine absorbs the UV light at a wavelength of about 200 nm.
Positively charged R group (Basic amino acids):
Positively charged amino acids act as proton donors. This is because of positive charge in its side chain. For example, arginine has a positive charge in the guanidine group and histidine in the imidazole ring. Histidine has a nearly neutral PH which means uncharged, but pKa shows a slight positive. Therefore it is classified as positively charged amino acids. Lysine (K) is also a positively charged amino acid.
Negatively charged R group (Acidic amino acids):
This class of amino acids act as the proton acceptor. Aspartate and glutamate have a negative charge at pH = 7.0. These two have the second carboxyl group.
Now we correlate the things we studied. If another amino acid replaces one amino acid, we should consider its properties and analyze the protein function. For example,
Questions related to importance of every amino acid in a protein:
1. Which of the amino acid substitutions is likely to cause the most significant change in protein conformation and function? (GATE 2015)
(A) Phe to Ile (B) Ser to Thr
(C) Gln to Tyr (D) Glu to Val
ANS: (D) Glu to Val
Explanation: The given option changes in aromatic amino acids to aliphatic, or vice versa, causes a change in protein function but comparatively Hydrophobic to hydrophilic amino acids substitution, or vice versa causes the largest chain in protein, the answer is option “D.”
2. Which amino acid has the highest probability to be present on the surface of a globular protein in an aqueous environment? (GATE 2015)
(A) Ala (B) Val
(C) Arg (D) Ile
ANS: (C) Arg
Explanation: Arginine is hydrophilic (water-loving) amino acid. So, Arg has to be found on the surface of the globular protein in an aqueous environment.
Peptide bond:
The amino group (NH2) attached to the alpha carbon (α- carbon) of one amino acid covalently bonded with the carboxyl group (COOH) of another amino acid. During this process, one H2O molecule will be eliminated. It happens with two different or same amino acids, and it is an example of an amide bond.
<a href=’https://www.freepik.com/vectors/design’>Design vector created by macrovector – www.freepik.com</a>
Discover more from Aristoscienceworld
Subscribe to get the latest posts sent to your email.