In the past few months, most heard words are covid-19 pandemic, Quarantine, lockdown, and “vaccines”. In this pandemic situation, everyone wishes to get vaccines against Covid-19. Such vaccines are now more critical for people to lead an everyday life. In 1796, the first vaccine was developed by Edward Jenner. That is the smallpox vaccine.
Moreover, this pandemic causes people to get frustrated. We can’t do anything until the production of successive effective vaccines. People wore masks, used hand sanitizer and kept a social distance to prevent infection.
What are vaccines and the process of vaccination?
Production of vaccines is like the proverb “Diamond cuts Diamond”. In short, vaccines are a suspension of avirulent (inactivated) pathogens that will cause our body to produce active immunity against those pathogens. Vaccination is the ingestion of vaccines into the human bloodstream. Pathogens are any microorganism that causes severe and contagious diseases to the human body. So, all conditions do not accept the same vaccines. Hence, different types of vaccines are used for different diseases.
Classification of vaccines:
1. Whole-cell vaccine: uses the whole pathogen for vaccine production.
2. Live attenuated vaccine: it is the same as whole-cell for a vaccine in a weakened form.
3. Inactivated vaccine: uses a whole cell in the form of an inactive vaccine.
4. Subunit vaccine: uses fragments (protein) or products of the pathogens for the vaccine.
5. Polysaccharides and conjugated vaccine: for vaccine production, sugar capsules (bacteria) and polysaccharides linked to a protein (antibody) are used.
6. Toxoid vaccine: toxins derived from exotoxins secreted by bacteria converted to toxoid by formalin or other heat treatment, and it is used when the leading cause of illness is a bacterial form. E.g., Diphtheria, Tetanus.
7. DNA vaccines: uses pathogen DNA which is essential for the antibody of that pathogen.
8. Recombinant DNA vaccine: engineered pathogen DNA infused in microbial vectors and transfecting to host cells.
Live attenuated vaccine:
It uses an avirulent form of live pathogens. Virulent forms of pathogens have adverse effects on humans, while avirulent are safe for the human body. It has a chance of getting transformed from avirulent to virulent while processing. In the processing of virulent to an avirulent pathogen, that pathogen is treated in cell culture. No booster shot(multiple doses in a regular interval) is needed for this vaccine. The challenge is that the mutation creates virulence of that pathogen. It can be reversible. It produces cellular and humoral immune reactions in the human body. In this vaccine, live cells can replicate in host cells and aid in producing more memory immune cells by providing continuous antigenic stimulus.
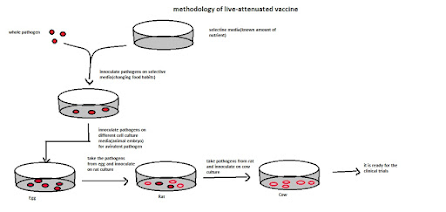
Methodology:
Modifying with host specificity.
1. Take the whole pathogen.
2. Plated on selective media for changing the gene sequence of food habit (known amount of nutrient)
3. Take the pathogen in the particular media and inoculate it in the cell culture media (animal embryo) to make it virulent to avirulent.
4. Make serial inoculation of the pathogen available for the human body (makes it avirulent).
Live attenuated vaccines are: BCG, OPV, Measles, Rubella, Rotavirus, Influenza.
Inactivated vaccines:
It uses whole inactivated or killed pathogens. There is no risk of getting diseases using this vaccine. The pathogen is treated with heat, γ-irradiation, acetone, phenol, β-propiolactone or formaldehyde to inactivate the vaccine. It causes humoral immune responses to the human body. It’s not reversible. The challenge is, it has weakened immune reactions due to no more extended availability of antigenic stimulus. It cannot replicate in host cells. It required a booster shot for effective immune responses.
Some of the inactivated vaccines are Hepatitis A, Flu, Polio, Rabies.
Subunit vaccine:
It is a non-reversible vaccine. Fragments or products of pathogens are used for vaccine production. Mostly surface protein of the pathogen is used. Challenges are that antigen selection is crucial, and it doesn’t induce many immune responses because of the only presence of antigen. It has fewer stranger immune responses than live attenuated vaccines(LAVs), mostly immune response based on the antigen used, and so Booster is required. Due to a lack of immunogenicity, it is necessary to use suitable adjuvants. It increases the effectiveness of vaccine adjuvants, not a fragment of the pathogen. E.g., Detergent, non-pathogenic bacteria. Recombinant DNA technology or conventional biochemistry methods are used for this vaccine production.
Some of the subunit vaccines are hepatitis B, Acellular Pertussis, Hib, PCV-7.
Conjugate vaccine:
Sugar(weak antigen) is attached to the strong antigen (protein) for vaccine production. Potent antigen acts as a carrier of the sugar (polysaccharides). Sugar is covalently bound to the potent antigen. Then it triggers more robust immune responses for that weak antigen (sugar). This vaccine is more effective for infants or the immune system. This is because it elicits the humoral immune responses against that pathogen and can’t be reversible. Conjugate vaccines prevent the asymptomatic carriage of bacterial diseases and destroy encapsulated bacteria, and it may use a booster for vaccine production.
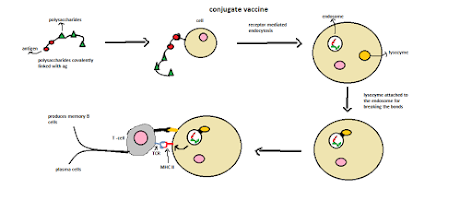
Principle:
1. Take the weak antigen (polysaccharides) covalently linked to the potent antigen.
2. After being injected into the body, it is engulfed by receptor-mediated endocytosis.
3. Lysozyme filled with hydrolytic acids is attached to the endosomes for breaking the interaction (peptide bonds) and MHC II molecule bound to the antigen.
4. TCR (T-cell receptor) attached to the MHC II molecule produces signals to immune organs and causes the production of memory cells.
Some of the conjugate vaccines are the tetramine vaccine, whole-cell vaccine, Haemophilus influenzae type b conjugate vaccines.
DNA vaccine:
The DNA sequence of important antigens is used for vaccine production. The challenges are that it has a practical level only. None of the DNA vaccines is approved for human injection. It is not reversible. In this, the immune responses focus on the antigen only. It has both humoral and cellular immune responses. It lacks immunogenicity. The DNA vaccines clinical trials are ongoing for influenza, hepatitis B and C, malaria, HPV.
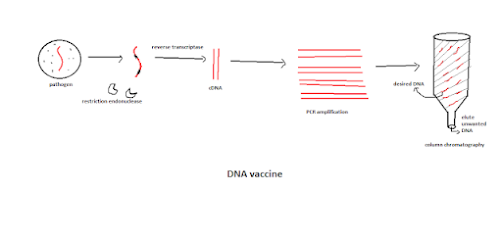
Methodology:
1. Take the RNA from the virus and cut the region of the gene of interest (encoded for immunogen or antigen) by a restriction endonuclease.
2. Make the RNA strand to cDNA using a primer.
3. Run it in a PCR(polymerase chain reaction) for amplification.
4. Finally, purifying using column chromatography.
Recombinant DNA vaccine:
Recombined pathogen DNA (encoding for antigen or immunogen) in microbial vectors are used for vaccine production. The difference between the DNA vaccine and the recombinant vaccine is that DNA vaccines use the antigen DNA and produce antigen in the host cells, while rDNA vaccines are processed and delivered the antigen in the invitro and injected into the vaccine.
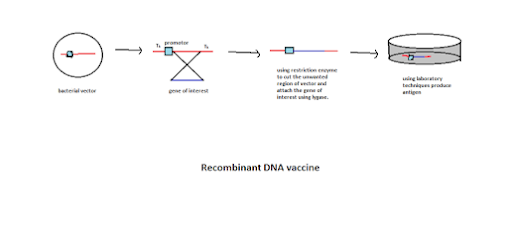
Methodology:
1. Take the gene of interest of the pathogen and take the bacterial vector.
2. The bacterial vector has the tk region on both ends, and the flanking part of the vector has the promoter.
3. Cut the vector region used for the pathogen gene interest with restriction of the endonuclease enzyme.
4. Using ligase enzyme, attach the gene of interest into the vector gene.
5. They are transfecting the vector into the cell culture medium. Amplify and produce antigen. Now the antigen is encoded by the desired gene of the pathogen.
Discover more from Aristoscienceworld
Subscribe to get the latest posts sent to your email.