Nitrogen is a chemical compound that is abundantly present in nature and it is an uncombined element. Nitrogen contributes about 78% of the total atmospheric gases. It is a significant constituent of amino acid, nucleic acid and Adenosine triphosphate (ATP). Over 3% of our body has N2, which is the fourth abundant element in our body. The deficiency causes the weakness of amino acids, leading to a lack of nucleic acid and ATP.
The nitrogen cycle comprises:
Nitrogen fixation, Ammonification, Nitrification, Assimilation and Denitrification.
Importance of Nitrogen:
A common amino acid contains hydrogen, carboxylic acid, an organic group and an amino group. Thus, nitrogen is a crucial constituent of amino acids that forms proteins which are building blocks of our body. Nitrogen is present in every cell of our body and serves as a building block of nucleic acids like DNA and RNA.
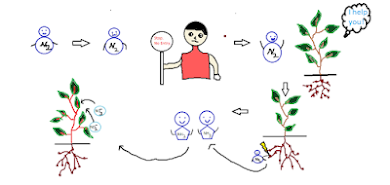
Why does N2 have to be fixed?
Unlike oxygen, we cannot uptake nitrogen through respiration. The main reason is that nitrogen is in the form of a molecule(N2) as it has a strong bond that is difficult to break and relatively inert. So, it has to be fixed for utilisation by organisms.
Nitrogen fixation:
Humans can inhale oxygen through respiration and result in the various metabolic pathways that produce ATPs and CO2. But intake of nitrogen is different. Since atmospheric N2 is molecular dinitrogen and, more importantly, has a trivalent bond, it requires high energy to break down. The definition of fixation is converting or fixing something into a utilisable form. Two methods can fix nitrogen: Nitrogen-fixing bacteria (primary process) and Lightening (minor process)
The atmospheric diatomic nitrogen is converted into Ammonia (NH3) by nitrogen-fixing bacteria present in the root nodules of plants. Nitrogen-fixing by lightning hardly breaks atmospheric nitrogen molecules.
Nitrogen-fixing bacteria:
These are Prokaryotic microorganisms that convert free nitrogen into fixed nitrogen, mainly ammonia.
There are two types of free-living bacteria:
Free-living:
Nitrogen-fixing bacteria that do not require a host and so live in soil or aquatic areas. Examples are Anabaena, Cyanobacteria, Nostoc, Azotobacter, Beijerinckia, Clostridium.
Mutualistic:
Nitrogen-fixing bacteria live in the root nodules of some plants, especially in leguminous plants, so they are called symbiotics. Examples are Rhizobium in leguminous plants, Frankia in dicotyledonous plants, Azospirillum in cereal grasses.
They are present in the soil and invade root hairs of plants resulting in the formation of root nodules with which Bacteria have a specialised enzyme called nitrogenase that converts the atmospheric nitrogen into two separate nitrogen atoms and makes them bind with hydrogen. i.e. Ammonia(NH3).
Nitrification:
Bacteria contain specific nitrifying enzymes, namely nitrite oxidoreductase and ammonia monooxygenase, which will convert the ammonia (substrate) into nitrates and nitrites (products), that takes it into the following process called assimilation.
Assimilation:
The uptake of nitrogen in nitrates(NO3–) by plant cells is called assimilation. The incorporated nitrogen is now used to synthesize proteins and nucleic acids. The plant cells utilise fixed nitrogen by incorporating into their corresponding outcomes like vegetables, fruits and leaves. Finally, nitrogen enter the food chain in green plants and that’s how we obtain nitrogen sources!
Denitrification:
The nitrogen taken from the atmosphere is retired back into the air by denitrification. Denitrifying bacteria like Pseudomonas and Paracoccus under anaerobic conditions perform a reduction process that reduces the nitrates into nitrogen gas, returning it to the atmosphere. The decomposition of animals and plants also results in denitrification using denitrifying bacteria, which completes the nitrogen cycle.
Discover more from Aristoscienceworld
Subscribe to get the latest posts sent to your email.